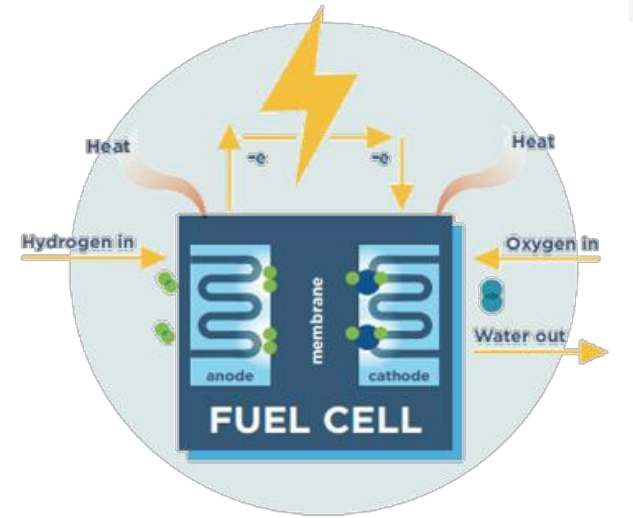
U4. Fuel Cells
- Electrochemical energy conversion device
- Continuous operation requires supply of fuel and oxidant and removal of water vapour, spent fuel, spent oxidant, inert residue and heat, etc.
- Fuel supplied at negative electrode (anode)
-
Oxidant supplide at positive electrode (cathode)
-
Exhaust -> if pure hydrogen used as fuel and pure oxygen as oxidant -> water vapor
- No cooling water is required
- current depends on the electrode area and can be increased by connecting several cells in parallel
Advantages
- quiet in operation as it is a static device
- less pollutant
- its conversion efficiency is more due to direct single stage energy conversion
- fuel cell plants are compact and require less space
- availability of choice from large number of possible fuels
- no cooling water is needed as required in condenser of a conventional steam plant. The heat generated can be easily removed and discharged to atmosphere or used locally
- can be installed near point of use, thus transmission and distribution losses are avoided
Classification of Fuel Cells 1. Based on type of Electrolyte 1. Solid Oxide Fuel Cell (SOFC) 2. Molten Carbonate Fuel Cell (MCFC) 3. Alkaline Fuel Cell (AFC) 4. Phosphoric Acid Fuel Cell(PAFC) 5. Solid Polymer Fuel Cell (SPFC) 2. Based on the Types of the Fuel and Oxidant 7. Hydrogen - Oxygen fuel cell (pure) 8. Hydrogen rich gas - air fuel cell 9. Hydrazine - Oxygen/Hydrogen peroxide fuel cell 10. Hydrocarbon (gas) - air fuel cell 11. Hydrocarbon (liquid) - air fuel cell 12. Ammonia - air fuel cell 3. Based on Operating Temperature 14. Low -> below 150 C 15. Medium -> 150 to 250 C 16. High -> 250 to 800 C 17. Very High-> 800 to 1100 C 4. Based on Application Fuel Cell For 1. Space Applications 2. Vehicle Propulsion 3. Submarines 4. defense applications 5. Commercial Applications 5. Based on Chemical Nature of Electrolyte 1. Acidic electrolyte Type 2. Alkaline electrolyte Type 3. Neutral electrolyte Type
Phosphoric Acid Fuel Cell¶
![[Pasted image 20240421180114.png]]
- Platinum serves as best catalyst for both electrodes and used for premium fuel cells.
- a less expensive material such as nickel (for negative electrode) and silver (for positive electrode) is used wherever possible.
- Negative Electrode
$H_2 -> 2H^+ \ + \ 2e^-$
- Electrons originating at negative electrode flow through the external load to positive electrode.
- $H^+$ ions migrate from Negative electrode towards positive electrode through the electrolyte
- Positive Electrode
$1/2\ O_2 \ + \ 2H^+\ + 2e^- \ -> H_2O$
- The operating temperature of PAFC is 150 °C–200 °C
- During the usage of the cell, the catalyst gradually loses its activity.
- This loss of activity is often attributed to 'poisoning' (inactivation) of the catalyst by the impurities (mostly sulphur compounds) in the fuel.
Molten Carbonate Fuel Cell (MCFC)¶
- carbonate of alkali metals (Na, K or Li) in molten (liquid) phase is used as electrolyte
- This requires the cell operation at a temperature above melting points (i.e. about 600–700 °C) of the respective carbonates
- 600 deg C to 700 deg C temperature
- High temperature thus catalyst not required
- Porous Nickel used for electrodes
- Special Feature!
- During operation the cell oxidizes
Hydrogen -> Waterand '\(CO -> CO_2\)'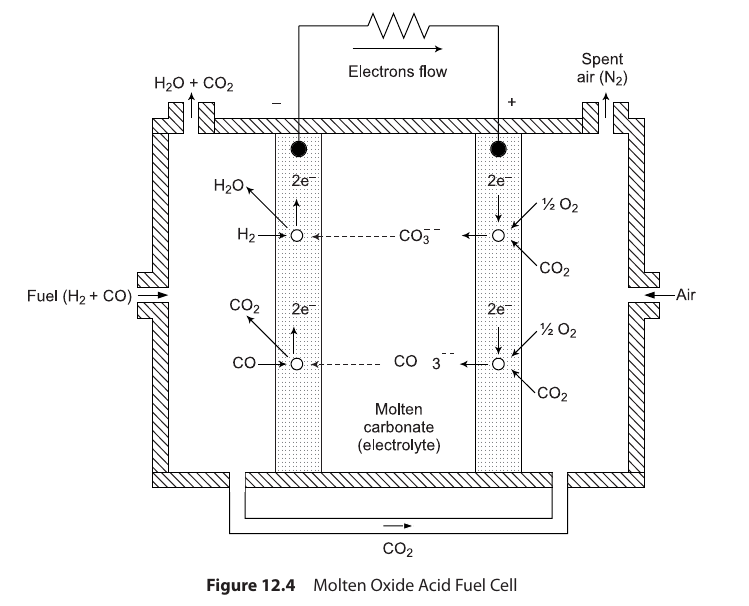
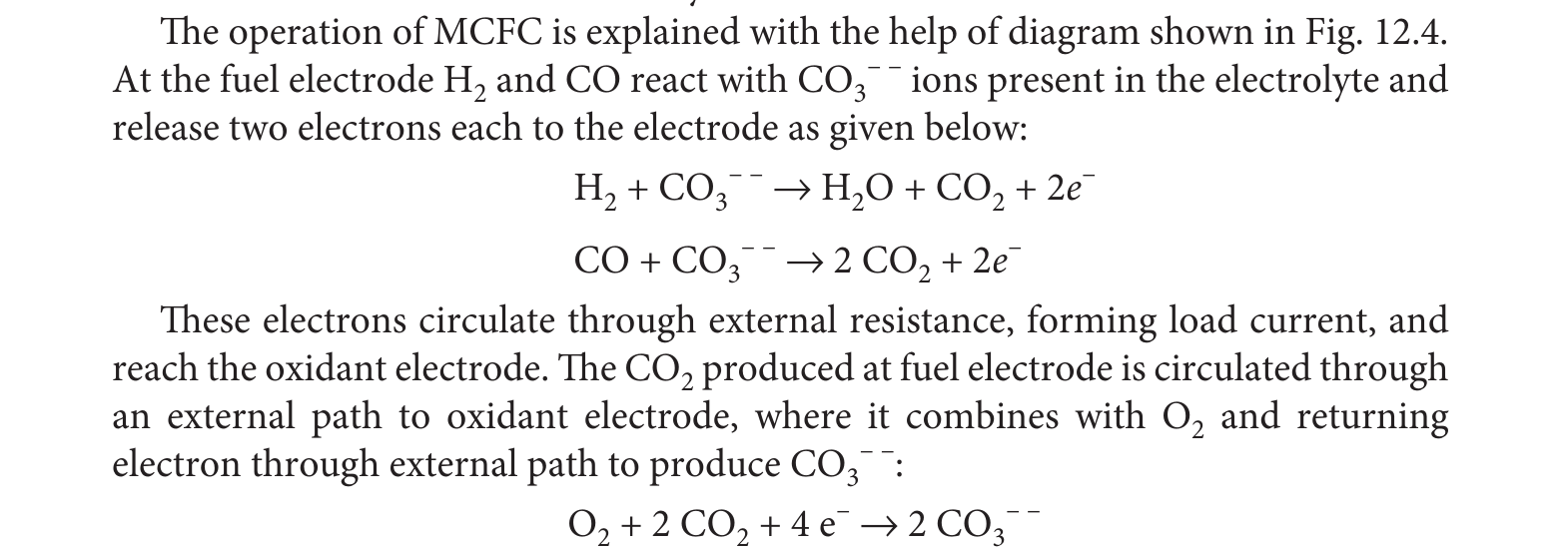
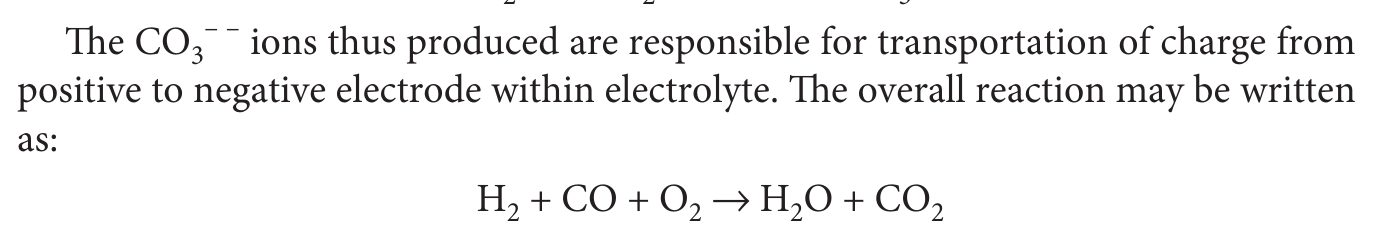
- During operation the cell oxidizes
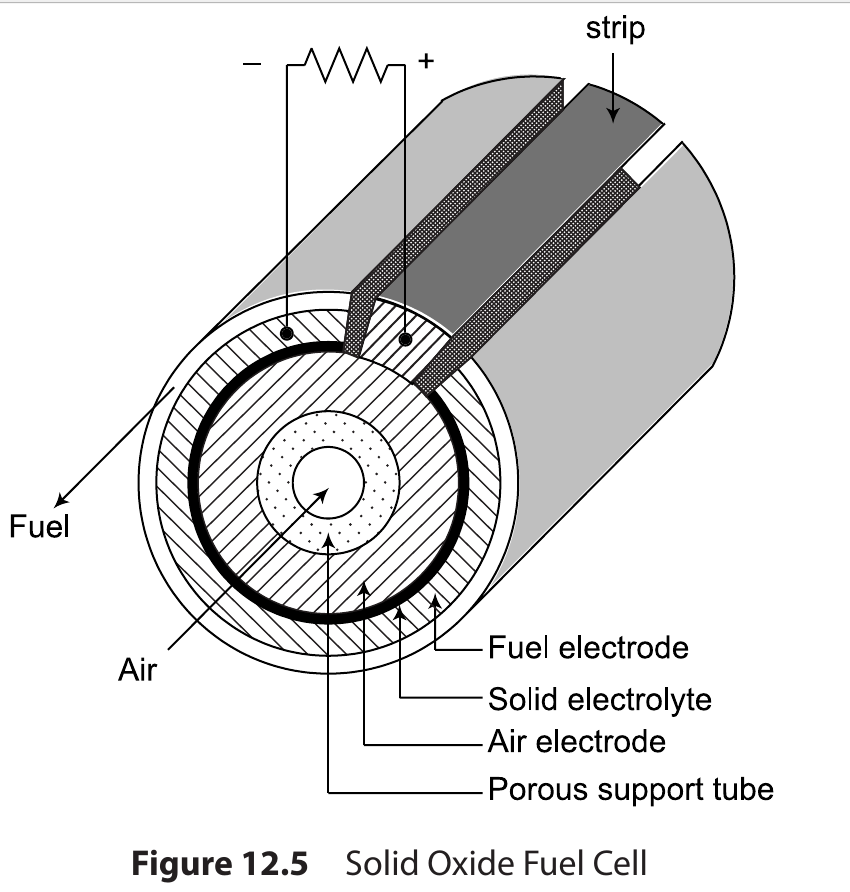
Solid Oxide Fuel Cell (SOFC)¶
- The negative electrode is made of porous nickel and positive electrode employs metal oxide, e.g. indium oxide.
- 600 to 1000 C operating range | High temperature thus no Catalyst
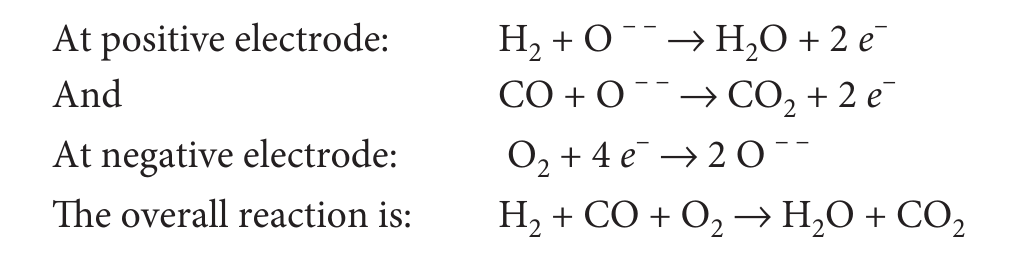
Direct Methanol Fuel Cell¶
- Chemical energy (Liquid Methanol) -> Electrical Energy
- liquid Methanol (\(CH_3OH\)) is oxidized in presence of water at anode to generate \(CO_2\), Hydrogen ions and electrons
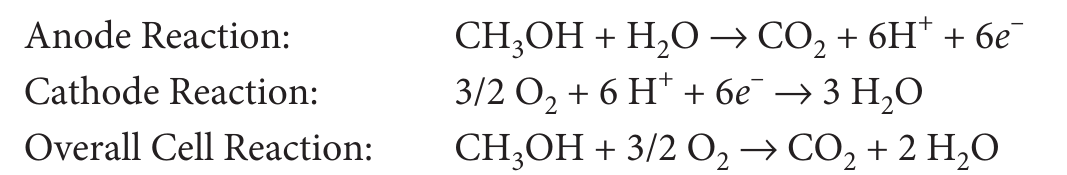
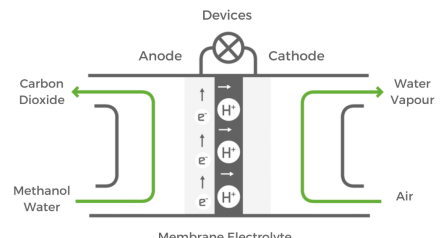 Caption - Membrane Electrolyte
Caption - Membrane Electrolyte

prev Unit: U3. Biomass next Unit : U5. Tidal Energy